A solution of a strong alkali at concentration 1 M (1 mol/L) has a pH of 14. Thus, in most problems that arise pH values lie mostly in the range 0 to 14, though negative pH values and values above 14 are entirely possible. Weak acid/base. Weak acids/bases only partially dissociate in water. Finding the pH of a weak acid is a bit more complicated.
What is the pH of a 0.025 M solution of KOH ? – YouTube
For the 3.0 M KOH solution find H^+, pH, pOH, and OH^-. Calculate the pH of a 0.0111 M KOH solution at the following temperature. 30^oC. Calculate the H+ of a 0.15 M KOH solution. Calculate the H+ in a 0.35 M KOH solution. Calculate the pH of each of the following solutions: a) 0.0010 M HCl b) 0.76 M KOH.

Source Image: toppr.com
Download Image
Jan 30, 2023Introduction. The pH of an aqueous solution is based on the pH scale which typically ranges from 0 to 14 in water (although as discussed below this is not an a formal rule). A pH of 7 is considered to be neutral. A pH of less than 7 is considered acidic. A pH of greater than 7 is then considered basic.

Source Image: youtube.com
Download Image
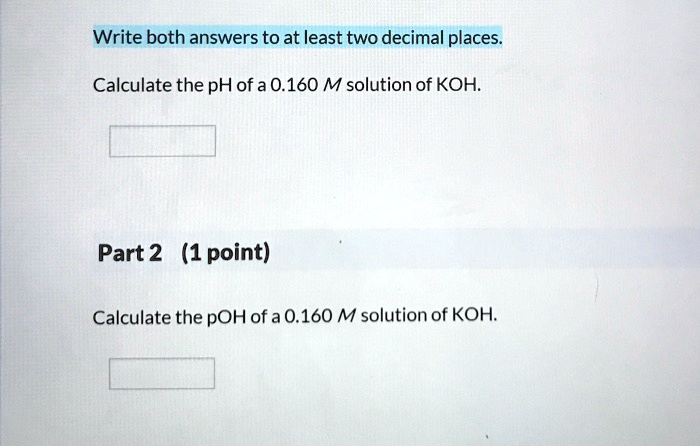
SOLVED: Calculate the pH of a 0.160 M solution of KOH. Part 2 (1 point) Calculate the pOH of a 0.160 M solution of KOH.
Concept explainers Question Calculate the pH of a 0.155 M solution of KOH. Calculate the pOH of a 0.155 M solution of KOH. Write both answers to at least two decimal places Expert Solution Trending now This is a popular solution! Step by step Solved in 3 steps with 1 images See solution Check out a sample Q&A here Knowledge Booster Learn more about

Source Image: numerade.com
Download Image
Calculate The Ph Of A 0.155 M Solution Of Koh
Concept explainers Question Calculate the pH of a 0.155 M solution of KOH. Calculate the pOH of a 0.155 M solution of KOH. Write both answers to at least two decimal places Expert Solution Trending now This is a popular solution! Step by step Solved in 3 steps with 1 images See solution Check out a sample Q&A here Knowledge Booster Learn more about
This online pH calculator is designed to determine the pH of an aqueous solution of a given chemical compound. You can select any acid or base from the list of chemicals, or use a known value for the dissociation constant K a or K b. The concentration of a substance can be specified either in moles or in units of mass per unit volume.
SOLVED: Write both answers to at least two decimal places. Calculate the pH of a 0.150 M solution of KOH. Calculate the pOH of a 0.150 M solution of KOH .
Question Part 1 Calculate the pH pH of a 0.155 M solution of KOH. Part 2 Calculate the pOH pOH of a 0.155 M solution of KOH. Solution Answered 8 months ago Create a free account to view solutions By signing up, you accept Quizlet’s Recommended textbook solutions Chemistry: The Molecular Nature of Matter and Change
Calculate the concentration of all species in a 0.155 M solu | Quizlet
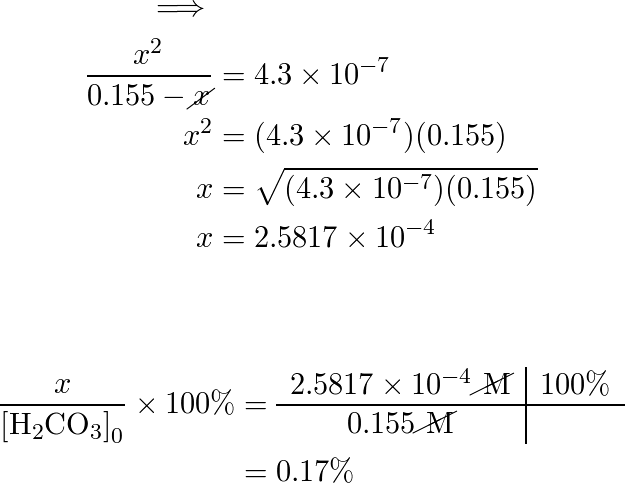
Source Image: quizlet.com
Download Image
What is the pH of 0.41 M of KOH? – Quora
Question Part 1 Calculate the pH pH of a 0.155 M solution of KOH. Part 2 Calculate the pOH pOH of a 0.155 M solution of KOH. Solution Answered 8 months ago Create a free account to view solutions By signing up, you accept Quizlet’s Recommended textbook solutions Chemistry: The Molecular Nature of Matter and Change

Source Image: quora.com
Download Image
What is the pH of a 0.025 M solution of KOH ? – YouTube
A solution of a strong alkali at concentration 1 M (1 mol/L) has a pH of 14. Thus, in most problems that arise pH values lie mostly in the range 0 to 14, though negative pH values and values above 14 are entirely possible. Weak acid/base. Weak acids/bases only partially dissociate in water. Finding the pH of a weak acid is a bit more complicated.

Source Image: youtube.com
Download Image
SOLVED: Calculate the pH of a 0.160 M solution of KOH. Part 2 (1 point) Calculate the pOH of a 0.160 M solution of KOH.
Jan 30, 2023Introduction. The pH of an aqueous solution is based on the pH scale which typically ranges from 0 to 14 in water (although as discussed below this is not an a formal rule). A pH of 7 is considered to be neutral. A pH of less than 7 is considered acidic. A pH of greater than 7 is then considered basic.
Source Image: numerade.com
Download Image
Solved Calculate [OH-], POH, and pH for each of the | Chegg.com
VIDEO ANSWER: When k, o h is placed into water and the rest’s water is not, everything is in a 1 to 1 to 1 ratio So, if we start with a substance that has a.150 molar, then this k– oh, is.150 molar. That means the concentration of each of the ion is
![Solved Calculate [OH-], POH, and pH for each of the | Chegg.com](https://media.cheggcdn.com/media/b4d/b4d17600-4104-4ce2-bc7f-a8dca92a5e66/phpCqC8Ou.png)
Source Image: chegg.com
Download Image
The `pH` of `0.01 (M) KOH` is `12`, if the temperature of the given `KOH` solution is increased – YouTube
Concept explainers Question Calculate the pH of a 0.155 M solution of KOH. Calculate the pOH of a 0.155 M solution of KOH. Write both answers to at least two decimal places Expert Solution Trending now This is a popular solution! Step by step Solved in 3 steps with 1 images See solution Check out a sample Q&A here Knowledge Booster Learn more about

Source Image: youtube.com
Download Image
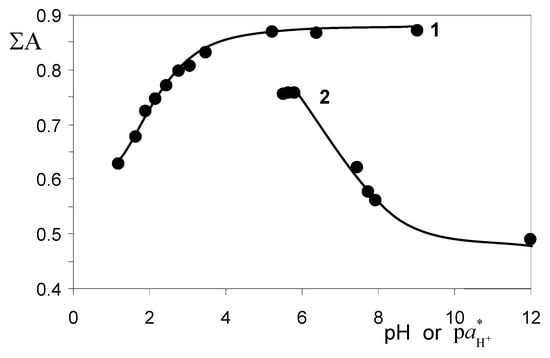
Colorants | Free Full-Text | Stability of Rhodamine Lactone Cycle in Solutions: Chain–Ring Tautomerism, Acid–Base Equilibria, Interaction with Lewis Acids, and Fluorescence
This online pH calculator is designed to determine the pH of an aqueous solution of a given chemical compound. You can select any acid or base from the list of chemicals, or use a known value for the dissociation constant K a or K b. The concentration of a substance can be specified either in moles or in units of mass per unit volume.
Source Image: mdpi.com
Download Image
What is the pH of 0.41 M of KOH? – Quora
Colorants | Free Full-Text | Stability of Rhodamine Lactone Cycle in Solutions: Chain–Ring Tautomerism, Acid–Base Equilibria, Interaction with Lewis Acids, and Fluorescence
For the 3.0 M KOH solution find H^+, pH, pOH, and OH^-. Calculate the pH of a 0.0111 M KOH solution at the following temperature. 30^oC. Calculate the H+ of a 0.15 M KOH solution. Calculate the H+ in a 0.35 M KOH solution. Calculate the pH of each of the following solutions: a) 0.0010 M HCl b) 0.76 M KOH.
SOLVED: Calculate the pH of a 0.160 M solution of KOH. Part 2 (1 point) Calculate the pOH of a 0.160 M solution of KOH. The `pH` of `0.01 (M) KOH` is `12`, if the temperature of the given `KOH` solution is increased – YouTube
VIDEO ANSWER: When k, o h is placed into water and the rest’s water is not, everything is in a 1 to 1 to 1 ratio So, if we start with a substance that has a.150 molar, then this k– oh, is.150 molar. That means the concentration of each of the ion is